The fossil calciferous skeletons of single-celled foraminifers are a beautiful history book with information on CO2-levels in the oceans of the distant past. "But if you want to fully understand that history, you must first understand exactly how these single-celled organisms build their skeletons."
That warning is issued by earth scientist Linda Dämmer in the dissertation she will defend today at Utrecht University.
Acidity and temperature
Foraminifers, or 'forams' for short, are single celled organisms that make a house of calcium carbonate to protect their cell from the outside world. That house also has ‘windows’ (foramina in Latin), hence the name. The skeletons consist not only of calcium carbonate (CaCO3) but also of traces of magnesium. "The amount of carbonate in the skeletons may reflect the amount of CO2 and the acidity of the ocean at that time," says Dämmer. "In addition, the amount of magnesium can tell a story about the temperature of the seawater. But that is not a simple black and white story," the researcher warns.
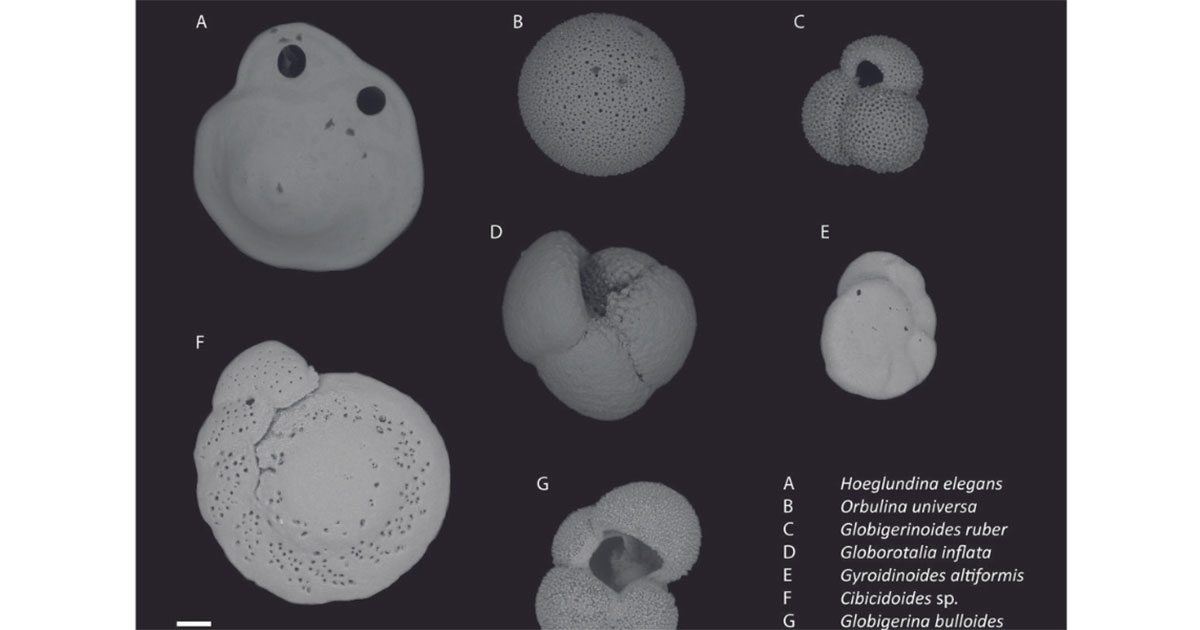 (Image credit: Royal Netherlands Institute for Sea Research)
(Image credit: Royal Netherlands Institute for Sea Research)
Light and dark
The amount of magnesium appears to be related not only to the temperature of the seawater, but also to the amount of daylight, Dämmer discovered during experimental culturing of her forams in the laboratory. If an organism starts building a new chamber to add to its calcium skeleton at dusk, it will contain more magnesium relative to calcium than in a conspecific, calcifying in continuous daylight at the same temperature. "So, a simple translation of the amount of magnesium to the temperature in which that organism lived is a simplification of reality," Dämmer said.
More foraminifers with more CO2
Some species of foraminifers are likely to benefit from the increasing amount of CO2 in the oceans, that results from the anthropogenic emissions. That growth may well continue until the amount of CO2 in our atmosphere reaches 700 parts per million (now it is 400 ppm). Above that, the acidity of the water will also become too high for these organisms to form calcium skeletons, meaning that even these resilient species will begin to struggle.
 Standing on the Italian Island of Panarea, watching smoke (and CO2) erupt from Mount Stromboli. (Image credit: Caitlyn Witkowski)
Standing on the Italian Island of Panarea, watching smoke (and CO2) erupt from Mount Stromboli. (Image credit: Caitlyn Witkowski)
Chemical understanding
Dämmer is not so much interested in the ecological consequences of an increase in one foraminifer or another. "In the food chains in the ocean you're probably not going to notice these changes. But to understand the complete accounting of CO2 and calcium in the oceans, it is very important to know exactly what these single-celled organisms are doing. In the open oceans, as much as half of the amount of calcium carbonate precipitated, consists of these tiny forams. In that respect, they can match coral reefs in other places in the oceans in terms of importance to ocean chemistry."



